( Fr- fluor/fluorite; Ger- Fluorit/Flußspat/Fluorkalzium;
Nor- flusspat/fluoritt; Rus- ![]() )
)
FLUORITE, CaF2.
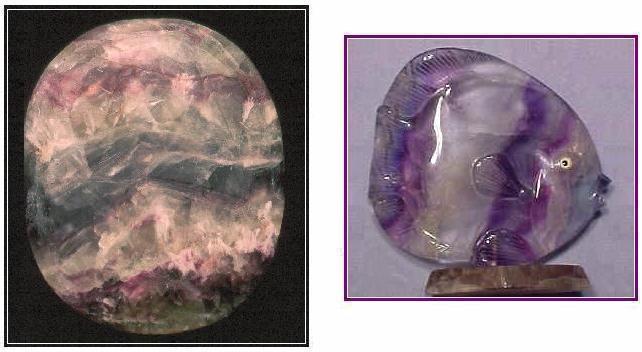
A. Fluorite (greater axis - 18 cm) honed oval from China. R.V. Dietrich collection. (© photo by Dick Dietrich)
B. Fluorite fish (height - ca. 11 cm) carved from banded fluorite from China. Southwestern Sales, Inc. (© photo by George Krismanits, www.southwesternsales.net)
DESCRIPTION:
Colors - reddish (rose to pink), yellowish,
green, bluish, purple, white and nearly colorless, commonly
color-banded or otherwise varicolored, in some pieces because of
diverse intensities or hues of only one color
H. 4
S.G. ~ 3.18
Light transmission - transparent to translucent
Luster - subvitreous to
vitreous
Breakage - four easy cleavages
Miscellany - much, but not all(!),
fluorite fluoresces under ultra violet radiation.
OTHER NAMES: (=fluorspar or even fluor)
USES: Snuff bottles, spheres, bowls, vases, figurines, paperweights, lamp bases, trinkets, urns, inlays, etc. Some particularly interesting items consist of matching pyramids made by sawing cleavage octahedra in two; however, these, as parts of large crystals or individual mineral grains, are not gemrocks. Pough (1996, p. 180) reports that "The Chinese make many fluorite carvings, which are marketed under the misleading name of 'green quartz.'"
OCCURRENCES: Several diverse occurrences, but especially as veins and nodular concretionary masses, many of which are in calcareous rocks; hydrothermal or pneumatolytic activities are usually said to account for their origin.
NOTEWORTHY LOCALITY: Blue John lead mine at Treak Cliff, near Castleton, Derbyshire, England; the Aspen Mine, near Silverton, San Juan County, Colorado; and Hardin County, Illinois.
REMARKS: The name fluorite is "from Latin
for to flow, since it melts
more easily than other minerals with which it was confused" (Mitchell,
1979). The fact that much fluorite emits electromagnetic
radiations (exhibited by visible light) when viewed under, for example,
ultraviolet light rays, led to the now widely used term fluor[spar]escence
to describe this property, which is exhibited by several natural and
man-made substances as well as by much (but not all!) fluorite.
Some fluorite in the marketplace has been irradiated to deepen its colors. In general, fluorite's easy cleavages make it less than desirable for several gemrock uses; to overcome this problem, some fluorite has been impregnated to strengthen it and make it less likely to break along any of its cleavages when it is submitted to carving or other fashioning processes.
The color of some fluorite fades with extended exposure to sunlight. Also, it has been reported that some fluorite expands and splits (cleaves) when heated, in some cases as the result of the mineral's being left in bright sunlight; unfortunately, such breakage may be intensified for fluorite that has been previously immersed in water, which is the way people frequently display it.
Color-banded fluorite, widely referred to as Bluejohn, from Derbyshire, England has been called "Britain's rarest gem." The widespread report that vases (etc.) fashioned from this fluorite were found among the early ruins of Pompeii, however, is now thought to be incorrect (Ford, 1955). The legend about the naming of Bluejohn, which is often told by guides at the Bluejohn Mine, is interesting, especially for anyone, like me, who is interested in language and word origins. Briefly, it is as follows: During the eighteenth century, when some of this fluorite was sent to France for jewelry, French craftsmen, apparently because of its typical color banding, referred to it as bleu-jaune (Fr.: blue-yellow). Subsquently, because of "standard linguistic laziness," that designation was corrupted to Bluejohn by the English (S. Fletcher, personal communication, 2002; see also img.cryst.bbk.ac.uk/BCA/bsg/Dblow). Photographs of several objects, including an especially noteworthy goblet, made from Blue John fluorite are shown on the web site: www.bluejohn-cavern.co.uk. The goblet is described as made by A.E. Ollenershaw (d) and "presented to H.R.H. The Duke of Edinburgh on the occasion of the opening of the Chemistry Building, Umist [University of Manchester Institute of Science and Technology], 2nd May, 1968."
According to Lynne (1988), fluorite has become "a rapidly growing favorite of the metaphysical crystal-collector, ... [although] as a mystical gem, ... [it] is a Johnny-come-lately." Indeed, she notes that different colors have been assigned different attributes -- e.g., red fluorite, is said to go "right to the root of the will to live and the physical vitality of the wearer."
The illustrated oval piece is especially attractive when placed under the moving water in our garden fountain. Its diversely colored zones exhibit an overall pattern that resembles a mountainous panorama in both transmitted and reflected lights.
Fluorite was named the
official
state mineral of Illinois in 1965; this status, however, was given
because
of its economic value as a nonmetallic ore, not because of its use as a
gemrock
or gemstone.
SIMULANTS:
***Calcium fluoride (i.e., synthetic fluorite) - this synthetic has been given many colors by doping it with different elements. One example, apparently strongly doped with Lanthanum, is alluded to by Liddicoat (1967). - [So far as gemrocks are concerned, such distinctions may not be required; this is so because the synthetics thus far produced comprise individual mineral entities rather than multigranular specimens (i.e., rocks). If, however, multigranular specimens, which should be termed rocks, are synthesized in the future and used as gemrocks, it seems only prudent to note here that distinquishing those synthetics from natural fluorite rocks will very likely require non-macroscopic procedures; at least some of them, however, may have specific gravities well outside the values for natural fluorite -- e.g., the one thought to be doped with Lanthanum has a S.G. of 4.89.].
REFERENCE: Lynne, 1988.
R. V. Dietrich © 2015
Last
update: 8 June 2005
web page created by Emmett Mason